How to update a .deb package with Mender using Raspberry Pi

Embedded Linux devices are very fragmented in their design and implementation. At the basic level, they all have the following components:
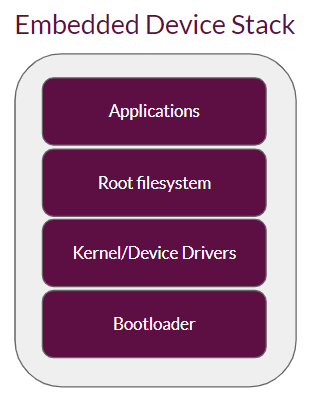
Applications implement the primary functions of a device; they are ultimately what makes the end user benefit from using the device. Therefore, they are the most frequently updated software stack to fix bugs and to add new features. They could also be updated as part of the root filesystem, also referred to as system updates, but it is preferred to be placed in a separate filesystem. This enables targeted application-level updates, which can be just a few kilobytes in size (compared to potentially hundreds of megabytes for root filesystem-level updates), with much lower bandwidth usage, faster updates and more frequent deployments.
Mender uses a framework called Update Modules to enable application-based updates and comes with support for these types of updates out-of-the-box:
- Packages (deb and rpm)
- Containers (docker)
- Files (directory copy/sync)
Today Debian is one of the most popular operating systems for connected devices. For users who don’t want the extra overhead and complexity associated with containers, updating Debian based systems often is as simple as updating a new version of a software package, or simply copy over new versions of specific files.
Learn more in this tutorial on how to use Deb Update Module in Mender to update a Debian package using a Raspberry Pi 3.
Recent articles
New Mender experimental AI-enabled feature
Mender in 2025: A year in review with compliance, security, and AI-driven growth
What’s new in Mender: Enhanced delta updates and user experience
Learn why leading companies choose Mender
Discover how Mender empowers both you and your customers with secure and reliable over-the-air updates for IoT devices. Focus on your product, and benefit from specialized OTA expertise and best practices.